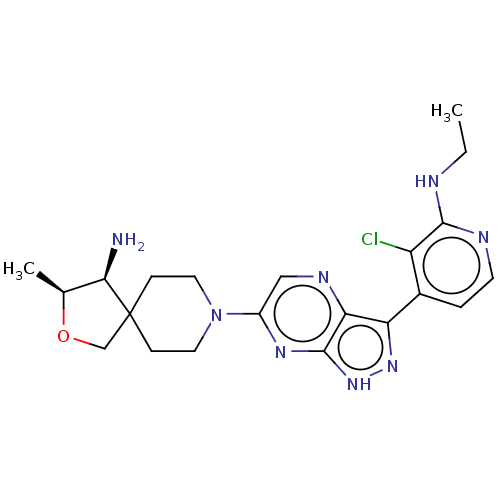
BDBM50588243 CHEMBL5183827
SMILES CCNc1nccc(-c2n[nH]c3nc(cnc23)N2CCC3(CO[C@@H](C)[C@H]3N)CC2)c1Cl
InChI Key InChIKey=ZNUSABOZHDKQTJ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50588243
Found 3 hits for monomerid = 50588243
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of N-terminal his6-tagged wild type recombinant human SHP2 (1 to 597 residues) expressed in Escherichia coli BL21 (DE3) cells using fluoro...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human SHP2 assessed as downregulation of PERK level in human KYSE520 cells incubated for 2 hrs by Alpha screen assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of hERG ion channel incubated for 3 hrs by fluorescence polarization assayMore data for this Ligand-Target Pair