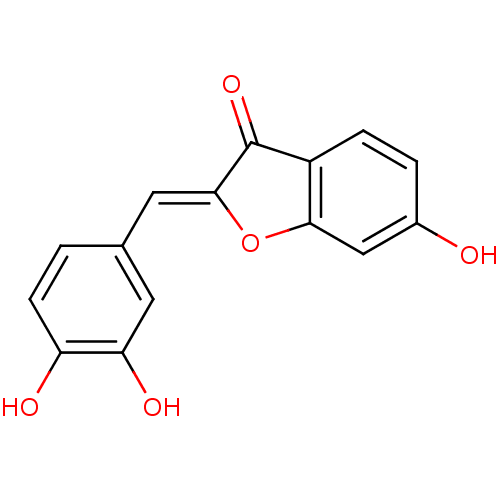
BDBM50580 (2Z)-2-(3,4-dihydroxybenzylidene)-6-hydroxy-coumaran-3-one::(2Z)-2-[(3,4-dihydroxyphenyl)methylidene]-6-hydroxy-1-benzofuran-3-one::(2Z)-2-[(3,4-dihydroxyphenyl)methylidene]-6-hydroxy-3-benzofuranone::(2Z)-2-[[3,4-bis(oxidanyl)phenyl]methylidene]-6-oxidanyl-1-benzofuran-3-one::MLS000863576::SMR000440743::SULFURETIN::cid_5281295
SMILES Oc1ccc2C(=O)\C(Oc2c1)=C\c1ccc(O)c(O)c1
InChI Key InChIKey=RGNXWPVNPFAADO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 38 hits for monomerid = 50580
Found 38 hits for monomerid = 50580
Affinity DataEC50: 424nMAssay Description:UNMCMD Assay Overview: Assay Support: 1 R03 MH087406-01A1 Project Title: Identification of broad-spectrum antifungal efflux pump inhibitors ...More data for this Ligand-Target Pair
Affinity DataIC50: 850nMAssay Description:Inhibition of human AURORA-AMore data for this Ligand-Target Pair
Affinity DataIC50: 950nMAssay Description:Inhibition of human TEKMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+3nMAssay Description:Inhibition of recombinant GST-tagged DRAK2 (unknown origin) autophosphorylation after 2 hrs by ADP-glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human CDK5More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Human)
Emory University
Curated by PubChem BioAssay
Emory University
Curated by PubChem BioAssay
Affinity DataIC50: 1.53E+3nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer...More data for this Ligand-Target Pair
Affinity DataIC50: 1.95E+3nMAssay Description:Inhibition of human GSK3betaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+3nMAssay Description:Inhibition of human ITKMore data for this Ligand-Target Pair
Affinity DataIC50: 2.16E+3nMAssay Description:Inhibition of human CDK9More data for this Ligand-Target Pair
Affinity DataIC50: 2.33E+3nMAssay Description:Inhibition of human DAPK3More data for this Ligand-Target Pair
Affinity DataIC50: 2.44E+3nMAssay Description:Inhibition of human CDK6More data for this Ligand-Target Pair
Affinity DataIC50: 2.82E+3nMAssay Description:Inhibition of human CDK4More data for this Ligand-Target Pair
Affinity DataIC50: 2.88E+3nMAssay Description:Inhibition of human BLKMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+3nMAssay Description:Inhibition of human DAPK2More data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Bovine)
National Academy of Sciences of Ukraine
Curated by ChEMBL
National Academy of Sciences of Ukraine
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Mixed-type inhibition of bovine milk xanthine oxidase assessed as enzyme-substrate-inhibitor complex using varying levels of xanthine as substrate by...More data for this Ligand-Target Pair
Affinity DataIC50: 4.16E+3nMAssay Description:Inhibition of human recombinant MAOA using kynuramine as substrate preincubated for 30 mins followed by substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.26E+3nMAssay Description:Inhibition of human JAK3More data for this Ligand-Target Pair
Affinity DataIC50: 4.63E+3nMAssay Description:Inhibition of human SYKMore data for this Ligand-Target Pair
Affinity DataIC50: 5.85E+3nMAssay Description:Inhibition of human DRAK1More data for this Ligand-Target Pair
Affinity DataIC50: 6.02E+3nMAssay Description:Inhibition of human BTKMore data for this Ligand-Target Pair
Affinity DataIC50: 9.32E+3nMAssay Description:Inhibition of human JAK2More data for this Ligand-Target Pair
Affinity DataEC50: 9.63E+3nMAssay Description:Keywords: STK33 Kinase, Non-ATP Competitive Inhibitor Assay Overview: Purified STK33 Kinase is preincubated with potential inhibitors and allowed to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+4nMAssay Description:Inhibition of human DAPK1More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase 1(Rat)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.97E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA...More data for this Ligand-Target Pair
Target26S proteasome non-ATPase regulatory subunit 14(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.38E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA...More data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
Universidad De Buenos Aires
Curated by ChEMBL
Universidad De Buenos Aires
Curated by ChEMBL
Affinity DataIC50: 2.96E+4nMAssay Description:Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.96E+4nMAssay Description:Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
Universidad De Buenos Aires
Curated by ChEMBL
Universidad De Buenos Aires
Curated by ChEMBL
Affinity DataIC50: 2.96E+4nMAssay Description:Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.62E+4nMAssay Description:Inhibition of Influenza A Jiangsu/10/2003 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+4nMAssay Description:Inhibition of CDK5/p25 (unknown origin) after 30 mins by SDS-PAGE analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.12E+4nMAssay Description:Inhibition of Influenza A Jinan/15/90 H3N2 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 5.29E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.63E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, germ cell type(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.72E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of human recombinant MAOB using benzylamine as substrate preincubated for 30 mins followed by substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.37E+5nMAssay Description:Inhibition of bovine liver arginase 1 using L-arginine as substrate incubated for 60 mins by OPA-p colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.99E+5nMAssay Description:Inhibition of ICR mouse brain AChE using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition by modified Ellman'...More data for this Ligand-Target Pair