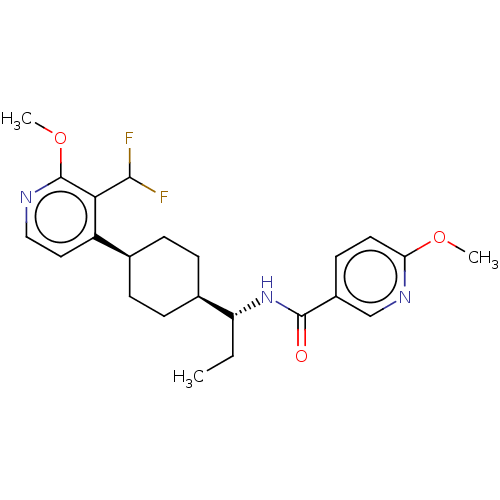
BDBM50571879 CHEMBL4848094
SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C(F)F)[C@@H](CC)NC(=O)c1ccc(OC)nc1
InChI Key InChIKey=VUXXMKIAYMJEHC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50571879
Found 8 hits for monomerid = 50571879
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Mouse)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by recombinant murine IFNgamma stimulation and measured af...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS-stimulated human whole blood preincubated for 4 hrs followed by IFNgamma/LPS stimulation and incubated for 18 hrs ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.90E+3nMAssay Description:Transactivation of PXR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+3nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 4.01E+3nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.74E+4nMAssay Description:Inhibition of hERG (unknown origin)More data for this Ligand-Target Pair