BDBM50571222 CHEMBL4871928
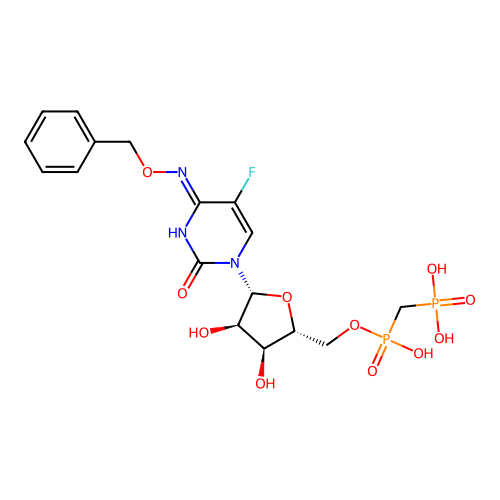
SMILES O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1cc(F)c(=NOCc2ccccc2)[nH]c1=O
InChI Key InChIKey=FFOWVPJTWFHPEY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50571222
Found 4 hits for monomerid = 50571222
TargetP2Y purinoceptor 6(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 549nMAssay Description:Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader methodMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 2.15E+4nMAssay Description:Binding affinity to human P2Y14 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 6(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 549nMAssay Description:Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by FLIPR methodMore data for this Ligand-Target Pair
Target5'-nucleotidase(Rat)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 85nMAssay Description:Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assayMore data for this Ligand-Target Pair