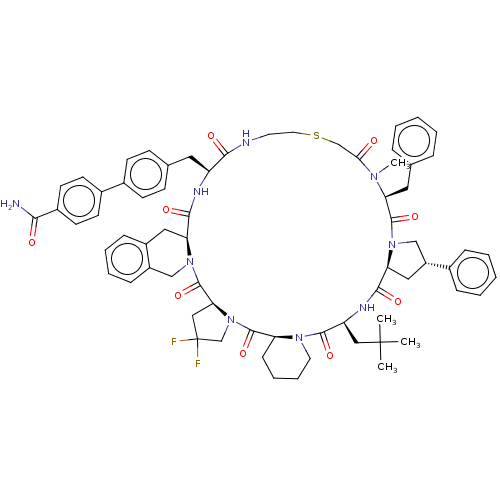
BDBM50566784 CHEMBL4872872
SMILES CN1[C@@H](Cc2ccccc2)C(=O)N2C[C@@H](C[C@H]2C(=O)N[C@@H](CC(C)(C)C)C(=O)N2CCCC[C@H]2C(=O)N2CC(F)(F)C[C@H]2C(=O)N2Cc3ccccc3C[C@H]2C(=O)N[C@@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)C(=O)NCCSCC1=O)c1ccccc1
InChI Key InChIKey=MYWJUCBOFWVIHA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50566784
Found 3 hits for monomerid = 50566784
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 770nMAssay Description:Inhibition of human NNMT expressed in HEK293 cells assessed as reduction in 1-methyl-nicotinamide formation measured after 24 hrs by RapidFire Mass s...More data for this Ligand-Target Pair