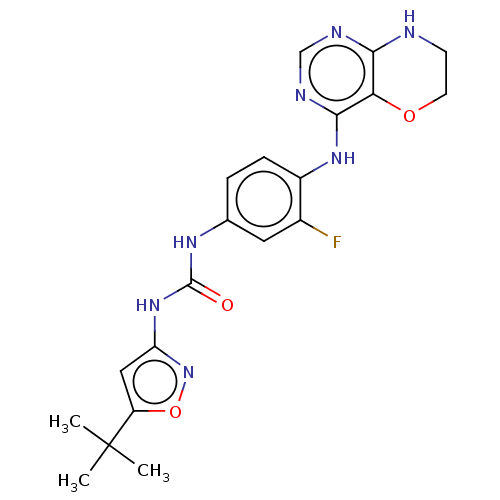
BDBM50563900 CHEMBL4784865
SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Nc3ncnc4NCCOc34)c(F)c2)no1
InChI Key InChIKey=WUZHXUJHAINKRH-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50563900
Found 3 hits for monomerid = 50563900
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant human RET V804L mutant using KKKVSRSGLYRSP as substrate incubated for 15 mins followed by Mg/ATP addition and measured afte...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of recombinant human RET V804M mutant using KKKVSRSGLYRSP as substrate incubated for 15 mins followed by Mg/ATP addition and measured afte...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of recombinant human RET using KKKSPGEYVNIEFG as substrate incubated for 15 mins followed by Mg/ATP addition and measured after 40 mins by...More data for this Ligand-Target Pair