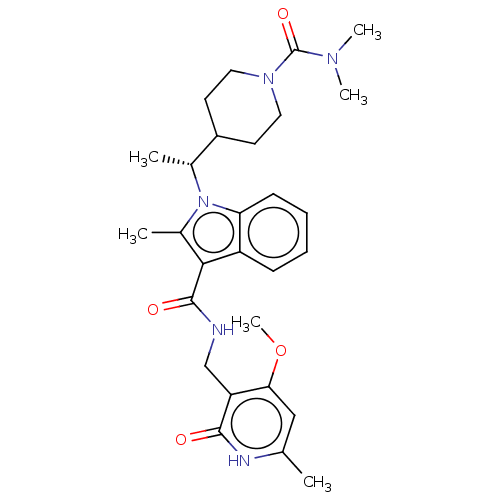
BDBM50563821 CHEMBL4780697
SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC2)C(=O)N(C)C)c2ccccc12
InChI Key InChIKey=AEFRRATWSMOUOK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50563821
Found 3 hits for monomerid = 50563821
Affinity DataIC50: 8.90nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataEC50: 930nMAssay Description:Inhibition of EZH2 in human HeLa cells assessed as inhibition of trimethylation of H3K27 after 72 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair