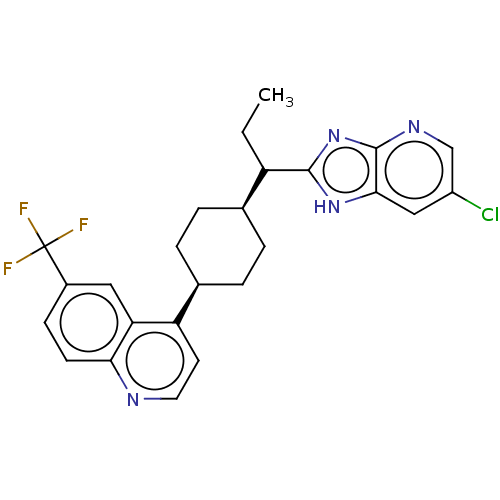
BDBM50559669 CHEMBL4756822
SMILES CCC([C@H]1CC[C@H](CC1)c1ccnc2ccc(cc12)C(F)(F)F)c1nc2ncc(Cl)cc2[nH]1
InChI Key InChIKey=SJAZDAYYSBXTSI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50559669
Found 10 hits for monomerid = 50559669
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of IDO1 in recombinant IFN-gamma induced human HeLa cells incubated for 18 hrs by fluorescence microplate reader assayMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Mouse)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of IDO1 in recombinant IFN-gamma induced mouse M109 cells incubated for 18 hrs by fluorescence microplate reader assayMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of IDO1 in IFN-gamma/LPS induced human whole blood assessed as tryptophan/kynurenine level measured after 18 hrs by RapidFire mass spectro...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.19E+3nMAssay Description:Transactivation of PXR (unknown origin) assessed as CYP450 inductionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.55E+3nMAssay Description:Inhibition of recombinant human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 4.28E+3nMAssay Description:Inhibition of recombinant human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+3nMAssay Description:Inhibition of recombinant human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 242nMAssay Description:Inhibition of recombinant human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+3nMAssay Description:Inhibition of recombinant human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of recombinant human CYP3A4More data for this Ligand-Target Pair