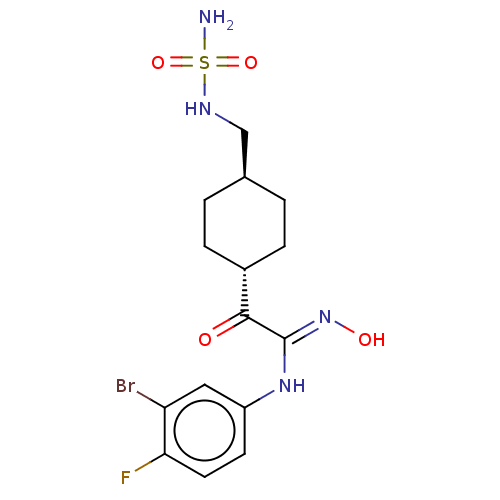
BDBM50549982 CHEMBL4789503
SMILES NS(=O)(=O)NC[C@H]1CC[C@@H](CC1)C(=O)C(\Nc1ccc(F)c(Br)c1)=N\O
InChI Key InChIKey=GFFNWLNMNQWQGN-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50549982
Found 3 hits for monomerid = 50549982
Affinity DataIC50: 21nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells using L-tryptophan as substrate measured after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 198nMAssay Description:Inhibition of human IDO1 expressed in Escherichia coli Rosetta using tryptophan as substrate incubated for 1 hr by methylene blue based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.87E+4nMAssay Description:Inhibition of human TDO expressed in Escherichia coli Rosetta using tryptophan as substrate incubated for 1 hr by methylene blue based assayMore data for this Ligand-Target Pair