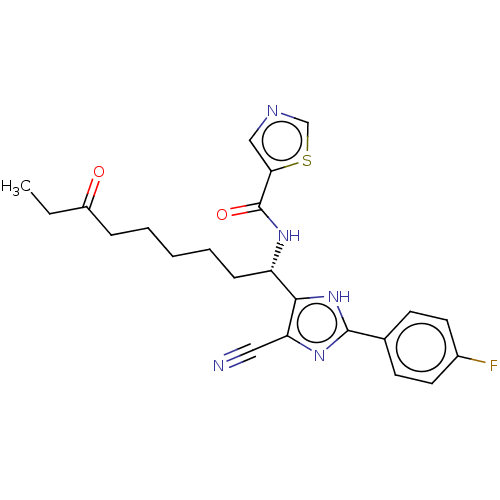
BDBM50546476 CHEMBL4760209
SMILES CCC(=O)CCCCC[C@H](NC(=O)c1cncs1)c1[nH]c(nc1C#N)-c1ccc(F)cc1
InChI Key InChIKey=CVDCDYPZZIBFOG-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50546476
Found 2 hits for monomerid = 50546476
Affinity DataEC50: 254nMAssay Description:Inhibition of Class 1 HDAC in VSV-G-pseudotyped HIV infected human 2C4 cell Jurkat model assessed as reactivation of HIV latency in presence of 0.1% ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.11E+3nMAssay Description:Inhibition of Class 1 HDAC in VSV-G-pseudotyped HIV infected human 2C4 cell Jurkat model assessed as reactivation of HIV latency in presence of 5% NH...More data for this Ligand-Target Pair