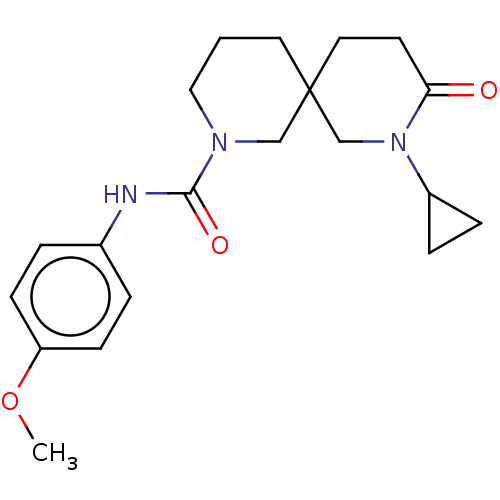
BDBM50545224 CHEMBL4633911
SMILES COc1ccc(NC(=O)N2CCCC3(CCC(=O)N(C3)C3CC3)C2)cc1
InChI Key InChIKey=IPXGBJBXPNSNFW-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50545224
Found 4 hits for monomerid = 50545224
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Human)
Orion Pharma
Curated by ChEMBL
Orion Pharma
Curated by ChEMBL
Affinity DataIC50: 1.55E+4nMAssay Description:Reversible inhibition of recombinant human KAT2 assessed as reduction in kynurenic acid formation using L-kynurenine as substrate by fluorescence ass...More data for this Ligand-Target Pair
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Human)
Orion Pharma
Curated by ChEMBL
Orion Pharma
Curated by ChEMBL
Affinity DataIC50: 1.55E+4nMAssay Description:Reversible inhibition of recombinant human KAT2 assessed as reduction in kynurenic acid formation using L-kynurenine as substrate by fluorescence ass...More data for this Ligand-Target Pair
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Human)
Orion Pharma
Curated by ChEMBL
Orion Pharma
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Reversible inhibition of recombinant human KAT2 assessed as reduction in kynurenic acid formation using L-kynurenine as substrate by fluorescence ass...More data for this Ligand-Target Pair
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Human)
Orion Pharma
Curated by ChEMBL
Orion Pharma
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Reversible inhibition of recombinant human KAT2 assessed as reduction in kynurenic acid formation using L-kynurenine as substrate by fluorescence ass...More data for this Ligand-Target Pair