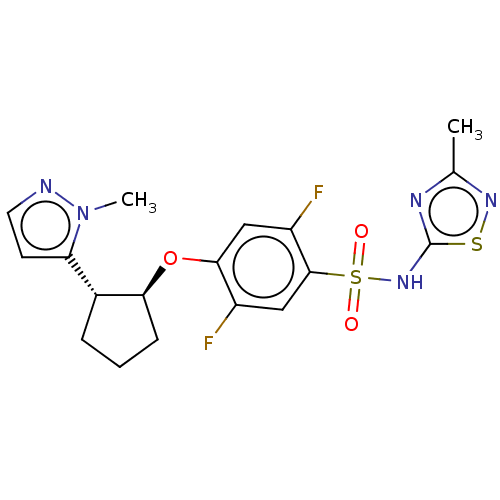
BDBM50543815 CHEMBL4641238
SMILES Cc1nsc(NS(=O)(=O)c2cc(F)c(O[C@H]3CCC[C@@H]3c3ccnn3C)cc2F)n1
InChI Key InChIKey=WGLIKNZYPIHIIX-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50543815
Found 4 hits for monomerid = 50543815
TargetSodium channel protein type 1 subunit alpha/subunit beta-1/subunit beta-2(Human)
Daiichi Sankyo
Curated by ChEMBL
Daiichi Sankyo
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NaV1.1/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology methodMore data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha/subunit beta-1/subunit beta-2(Human)
Daiichi Sankyo
Curated by ChEMBL
Daiichi Sankyo
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NaV1.5/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human ERG by Ionworks high-throughput electrophysiology methodMore data for this Ligand-Target Pair