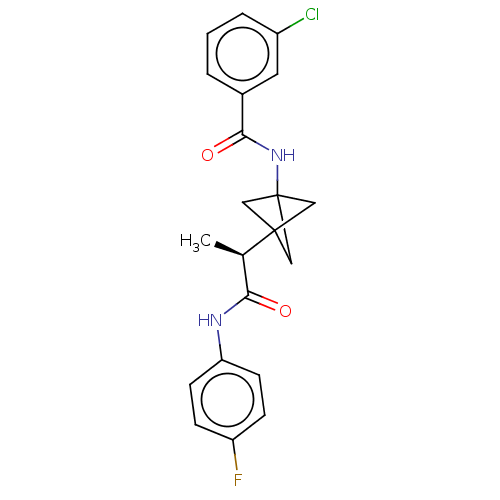
BDBM50543292 CHEMBL4632992
SMILES C[C@H](C(=O)Nc1ccc(cc1)F)C23CC(C2)(C3)NC(=O)c4cccc(c4)Cl
InChI Key InChIKey=ORFXTXYARYWSKK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50543292
Found 3 hits for monomerid = 50543292
Affinity DataIC50: 3.10nMAssay Description:Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 121nMAssay Description:Inhibition of IDO1 in human whole blood stimulated with IFNgamma/LPS using L-tryptophan/kynurenine as substrate incubated for 15 mins prior to IFNgam...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TDO in human SW48 cells using L-tryptophan as substrate incubated for 48 hrs by fluorescent microplate reader assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)