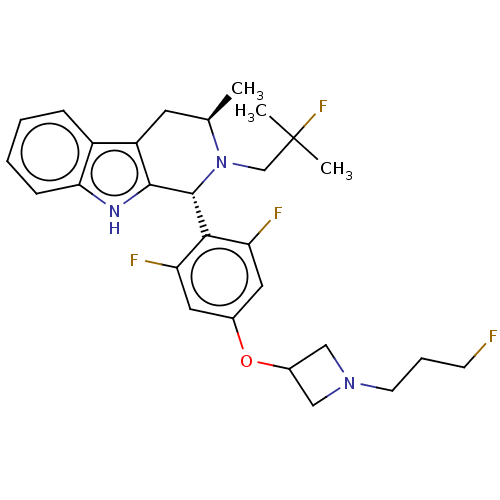
BDBM50542086 CHEMBL4649161::US20250114338, Example 101
SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OC2CN(CCCF)C2)cc1F
InChI Key InChIKey=BKHIBYHLBQEIQK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50542086
Found 4 hits for monomerid = 50542086
Affinity DataIC50: 0.0530nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0310nMAssay Description:Induction of ERalpha degradation in human T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair