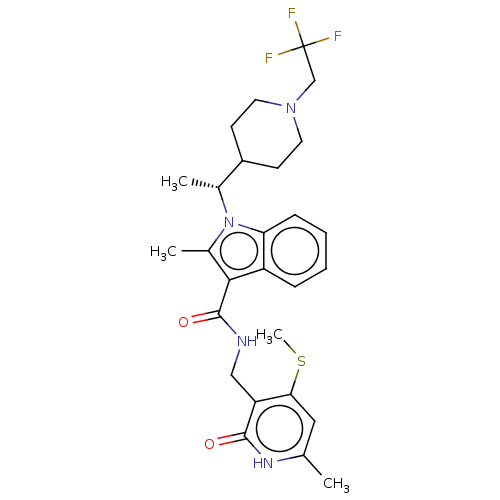
BDBM50541895 CHEMBL4639616::US11459315, Example 12
SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(F)(F)F)CC2)c2ccccc12
InChI Key InChIKey=JTUDWDHZMRIJHW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50541895
Found 3 hits for monomerid = 50541895
Affinity DataIC50: 0.180nMAssay Description:Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA...More data for this Ligand-Target Pair
Affinity DataEC50: 2.5nMAssay Description:Inhibition of EZH2 in human HeLa cells assessed as inhibition of trimethylation of H3K27 after 72 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame...More data for this Ligand-Target Pair