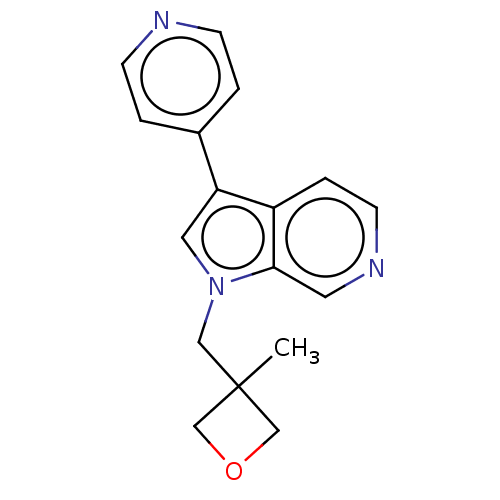
BDBM50538087 CHEMBL4644898
SMILES CC1(Cn2cc(-c3ccncc3)c3ccncc23)COC1
InChI Key InChIKey=SNTQSJLLVJJTOM-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50538087
Found 3 hits for monomerid = 50538087
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Human)
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 315nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Human)
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 2.85E+4nMAssay Description:Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting methodMore data for this Ligand-Target Pair