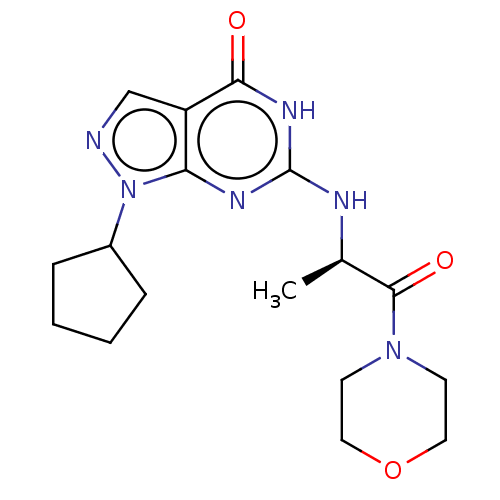
BDBM50521388 CHEMBL4544864
SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)N1CCOCC1
InChI Key InChIKey=GESQMVSOZXARLD-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50521388
Found 3 hits for monomerid = 50521388
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibition of recombinant human PDE9A2 catalytic domain (181 to 506 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 ...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE1B catalytic domain (10 to 487 residues) (unknown origin) using 3H-cGMP as substrate after 15 mins by liquid scintillation counting ...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of PDE9A2 (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate measured for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair