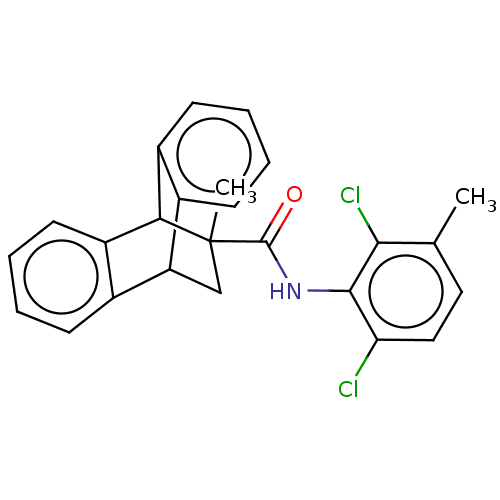
BDBM50518420 CHEMBL4555682
SMILES Cc1ccc(Cl)c(NC(=O)C2(C)CC3c4ccccc4C2c2ccccc32)c1Cl
InChI Key
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50518420
Found 3 hits for monomerid = 50518420
Affinity DataEC50: 56nMAssay Description:Positive allosteric modulation of human D1R expressed in HEK cells assessed as increase in dopamine-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Positive allosteric modulation of human D1R expressed in CHO cells assessed as increase in dopamine-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Positive allosteric modulation of rat D1R expressed in HEK cells assessed as increase in dopamine-induced cAMP accumulation relative to controlMore data for this Ligand-Target Pair