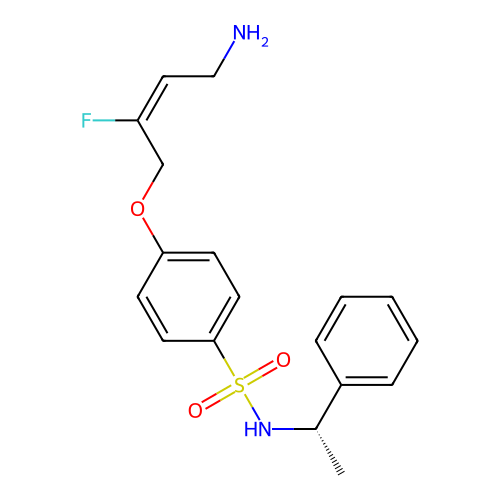
BDBM50505648 CHEMBL4562149
SMILES C[C@H](NS(=O)(=O)c1ccc(OCC(F)=C/CN)cc1)c1ccccc1
InChI Key InChIKey=ZVFVOMFIUGHFMF-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50505648
Found 3 hits for monomerid = 50505648
Affinity DataIC50: 2.51E+3nMAssay Description:Inhibition of recombinant human C-terminal His-tagged LOLX2 (1-774 residues) expressed in mouse myeloma cells using putrescine as substrate preincuba...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant human SSAO using benzylamine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair