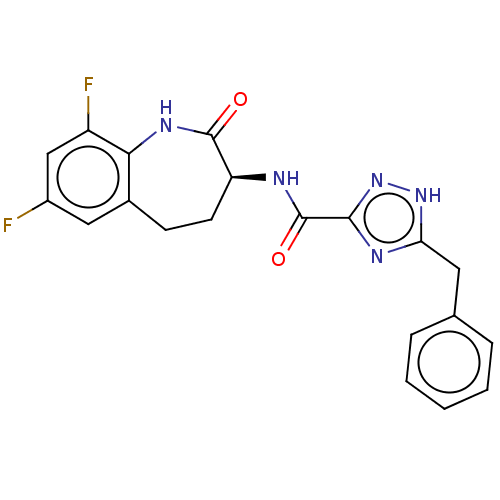
BDBM50502339 CHEMBL4452233::US20240270732, Control compound 2
SMILES Fc1cc(F)c2NC(=O)[C@H](CCc2c1)NC(=O)c1n[nH]c(Cc2ccccc2)n1
InChI Key InChIKey=ATQAGKAMBISZQM-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50502339
Found 10 hits for monomerid = 50502339
Affinity DataIC50: 0.400nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta cytokine overproduction measured at...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of RIP1 in human U937 cells assessed as reduction in necroptosis incubated for 24 hrs by cell titer-glo luminescent cell viability assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated necroptosis by measuring LDH release measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of pDEST8HisGSTTev-tagged human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated with enzyme for 1 hr ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of RIP1 in mouse L929 cells assessed as reduction in necroptosis incubated for 24 hrs by cell titer-glo luminescent cell viability assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of RIP1 in human whole blood assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta cytokine overproduction incubated f...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta mRNA overexpression measured at 21 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated necroptosis by measuring cellular ATP levels m...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Binding affinity to RIP1 (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20.6nMAssay Description:The compound of the present invention and the control compound were dissolved in the assay buffer (50 mM Hepes pH 7.5, 50 mM NaCl, 30 mM MgCl2, 1 mM ...More data for this Ligand-Target Pair