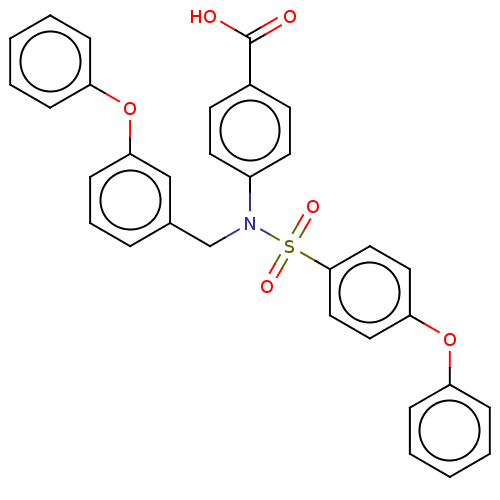
BDBM50468863 CHEMBL4287827
SMILES OC(=O)c1ccc(cc1)N(Cc1cccc(Oc2ccccc2)c1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1
InChI Key InChIKey=ZRRIGKURYVZVQJ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50468863
Found 3 hits for monomerid = 50468863
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 (DE3) using arachidonic acid as substrate preincubated for 10 mins followed b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of mPGES-1 isolated from microsomes of interleukin-1 beta-stimulated human A549 cells using PGH2 as substrate preincubated for 15 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of 5-LO in human neutrophils using arachidonic acid as substrate assessed as reduction in 5-LO product formation preincubated for 15 mins ...More data for this Ligand-Target Pair