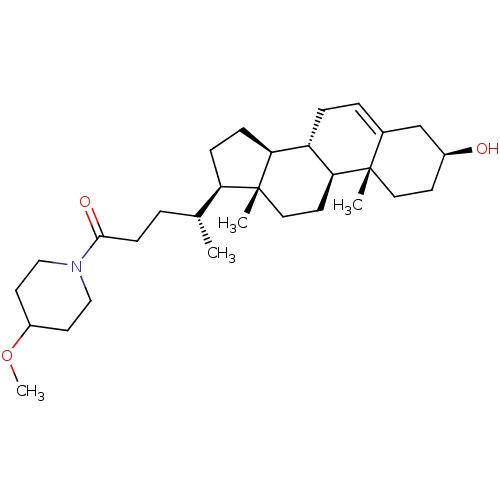
BDBM50465713 CHEMBL4277633::US20230340011, Example 94.
SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)OC
InChI Key InChIKey=PIZNXHJWRVOQEE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50465713
Found 3 hits for monomerid = 50465713
Affinity DataIC50: 125nMAssay Description:10,000 cells/well of the PathHunter U2OS SREBP-2 Nuclear Translocation Cell Line are plated in quadruplicate on 384 well plates in 20 μL of Assa...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Saint Louis University School of Medicine
Curated by ChEMBL
Saint Louis University School of Medicine
Curated by ChEMBL
Affinity DataEC50: 230nMAssay Description:Agonist activity at human LXRalpha expressed in HEK293 cells after 24 hrs by One-Glo luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 625nMAssay Description:PathHunter NHR CHO cells stably transfected with tagged full-length human LXRβ and a nuclear fusion protein containing Steroid Receptor Co-activ...More data for this Ligand-Target Pair