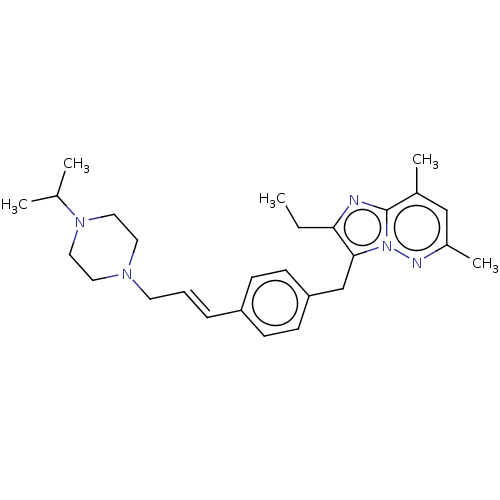
BDBM50450955 CHEMBL4205308
SMILES CCc1nc2c(C)cc(C)nn2c1Cc1ccc(\C=C\CN2CCN(CC2)C(C)C)cc1
InChI Key InChIKey=WKMOBAVFRIULDB-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50450955
Found 3 hits for monomerid = 50450955
TargetG-protein coupled receptor 4(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Displacement of [3H]dofetilide from recombinant human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Displacement of [3H]-R(-)-alpha-Methyl[imidazole-2.5(n)]histamine from human recombinant histamine H3 receptor expressed in CHOK1 cell membranes afte...More data for this Ligand-Target Pair