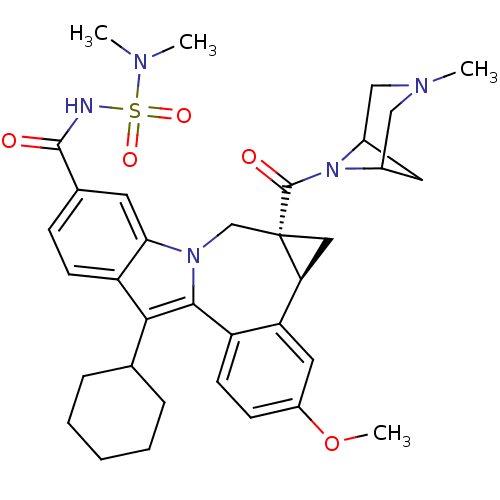
BDBM50448497 CHEMBL3126834
SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C
InChI Key
Data 5 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50448497
Found 5 hits for monomerid = 50448497
Affinity DataEC50: >4.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataEC50: >4.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataEC50: >4.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataEC50: >4.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPHMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+4nMAssay Description:Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair