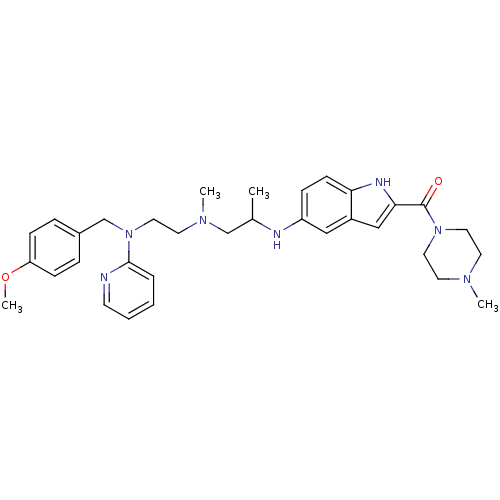
BDBM50419323 CHEMBL1910385
SMILES COc1ccc(CN(CCN(C)CC(C)Nc2ccc3[nH]c(cc3c2)C(=O)N2CCN(C)CC2)c2ccccn2)cc1
InChI Key InChIKey=YMLNOWNONRZEDQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50419323
Found 3 hits for monomerid = 50419323
Affinity DataKd: 12.6nMAssay Description:Antagonist activity at H1R in guinea pig ileum assessed as inhibition of histamine-induced muscle contraction after 15 minsMore data for this Ligand-Target Pair
Affinity DataKi: 214nMAssay Description:Displacement of [3H]mepyramine from human H1R expressed in Sf9 cells co-expressing RGS4 after 90 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.41E+4nMAssay Description:Displacement of [3H]histamine from human H4R expressed in Sf9 cells co-expressing RGS19, Galphai2 and Gbeta1gamma2 after 60 mins by liquid scintillat...More data for this Ligand-Target Pair