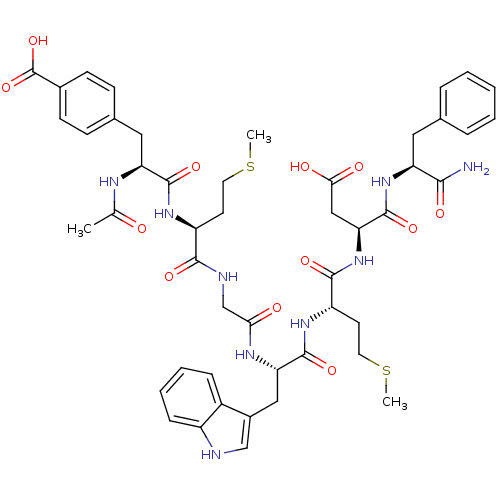
BDBM50406491 CHEMBL2079548
SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)C(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O
InChI Key InChIKey=KMBZDSDELKUPOP-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50406491
Found 2 hits for monomerid = 50406491
Affinity DataIC50: 30nMAssay Description:Inhibition of [3H]propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 62nMAssay Description:Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranesMore data for this Ligand-Target Pair