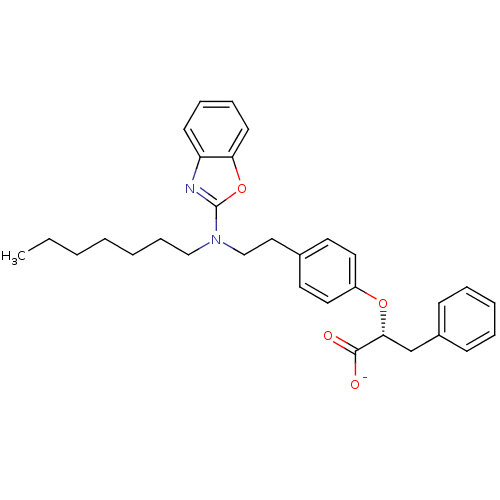
BDBM50401009 CHEMBL2206305
SMILES CCCCCCCN(CCc1ccc(O[C@H](Cc2ccccc2)C([O-])=O)cc1)c1nc2ccccc2o1
InChI Key InChIKey=CSFPOCDMBLMFTM-UHFFFAOYSA-M
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50401009
Found 2 hits for monomerid = 50401009
TargetPeroxisome proliferator-activated receptor alpha(Human)
Istituto Tumori&Quot;Giovanni Paolo Ii&Quot
Curated by ChEMBL
Istituto Tumori&Quot;Giovanni Paolo Ii&Quot
Curated by ChEMBL
Affinity DataEC50: 1.72E+3nMAssay Description:Agonist activity at human GAL4-fused PPARalpha ligand binding domain expressed in HepG2 cells after 20 hrs by luciferase reporter gene transactivatio...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Istituto Tumori&Quot;Giovanni Paolo Ii&Quot
Curated by ChEMBL
Istituto Tumori&Quot;Giovanni Paolo Ii&Quot
Curated by ChEMBL
Affinity DataEC50: 2.70E+3nMAssay Description:Agonist activity at human GAL4-fused PPARgamma ligand binding domain expressed in HepG2 cells after 20 hrs by luciferase reporter gene transactivatio...More data for this Ligand-Target Pair