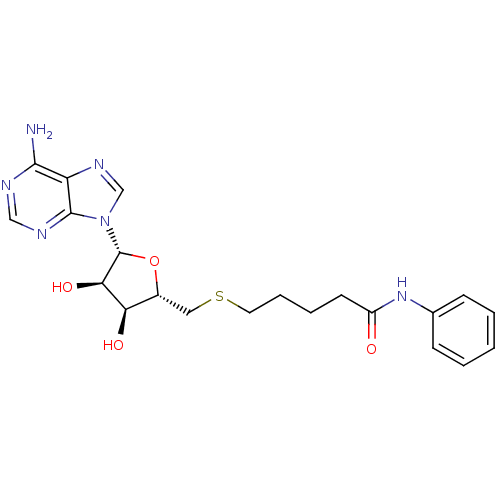
BDBM50397002 CHEMBL2170983
SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCCC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O
InChI Key InChIKey=BKKHEFHWVXCOJD-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50397002
Found 2 hits for monomerid = 50397002
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Human)
Baylor College of Medicine
Curated by ChEMBL
Baylor College of Medicine
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counterMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Human)
Baylor College of Medicine
Curated by ChEMBL
Baylor College of Medicine
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactorMore data for this Ligand-Target Pair