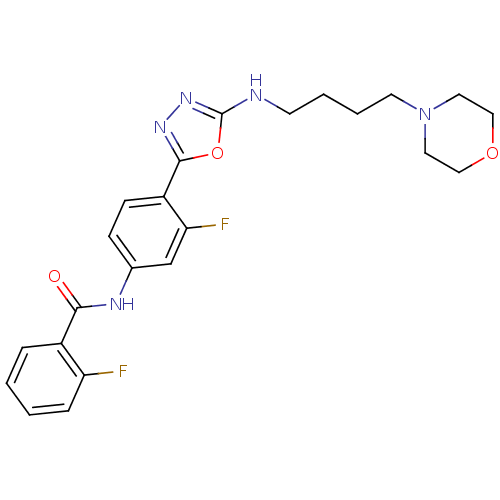
BDBM50382048 CHEMBL2022739
SMILES Fc1ccccc1C(=O)Nc1ccc(-c2nnc(NCCCCN3CCOCC3)o2)c(F)c1
InChI Key InChIKey=JZLFWWQOPQHUPT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50382048
Found 11 hits for monomerid = 50382048
Affinity DataEC50: 126nMAssay Description:Agonist activity at human alpha7 nAChR expressed in GH4C1 cells by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+4nMAssay Description:Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.86E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4 using diethoxyfluorescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+4nMAssay Description:Inhibition of CYP3A4 using 7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 6.31E+3nMAssay Description:Antagonist activity at alpha1 nAChRMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94E+3nMAssay Description:Antagonist activity at alpha4beta2 nAChRMore data for this Ligand-Target Pair