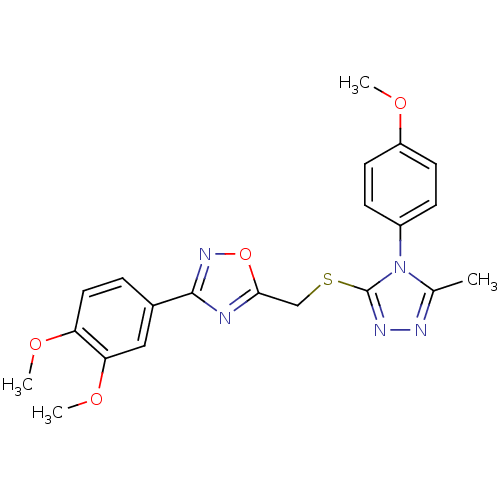
BDBM50380595 CHEMBL1867804
SMILES COc1ccc(cc1)-n1c(C)nnc1SCc1nc(no1)-c1ccc(OC)c(OC)c1
InChI Key InChIKey=HQPRJYQZDYZZOA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50380595
Found 9 hits for monomerid = 50380595
TargetPoly [ADP-ribose] polymerase tankyrase-2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of GST-tagged TNKS2P catalytic domain autoPARsylation measuring nicotinamide concentration after 2 hrs by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inhibition of mouse Wnt3A signaling in human HEK293 cells after 1 day by super top flash luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of PARP1 autoPARsylation measuring nicotinamide concentration after 2 hrs by LC-MS analysisMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of PARP2 autoPARsylation measuring nicotinamide concentration after 2 hrs by LC-MS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 375nMAssay Description:Stabilization of Axin2 in human SW480 cells after 24 hrs by sandwich ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair