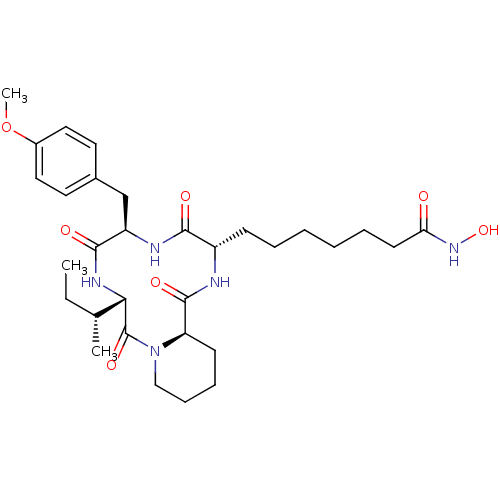
BDBM50366969 CHEMBL1790593
SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccc(OC)cc2)NC(=O)[C@H](CCCCCCC(=O)NO)NC(=O)[C@H]2CCCCN2C1=O
InChI Key InChIKey=IDNMSLGHIWCDPW-UHFFFAOYSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50366969
Found 1 hit for monomerid = 50366969
Affinity DataIC50: 4.80nMAssay Description:Inhibitory activity against histone deacetylases (HDAC) prepared from mouse melanoma B16/BL6 cells; Not testedMore data for this Ligand-Target Pair