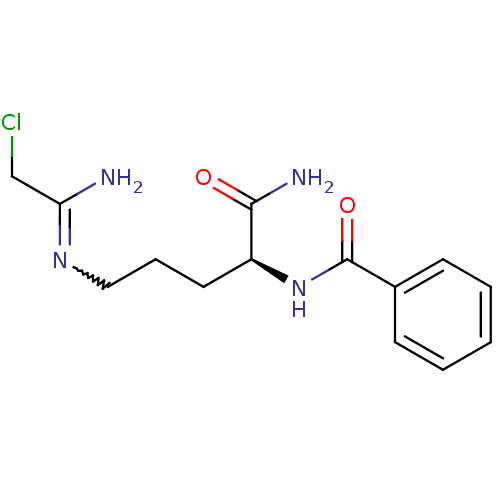
BDBM50355657 CHEMBL1910972::CHEMBL1962361
SMILES NC(=O)[C@H](CCCN=C(N)CCl)NC(=O)c1ccccc1
InChI Key InChIKey=BPWATVWOHQZVRP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50355657
Found 8 hits for monomerid = 50355657
Affinity DataIC50: 800nMAssay Description:Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of PAD4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Irreversible inhibition of PAD2 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataKi: 6.20E+4nMAssay Description:Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+5nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair