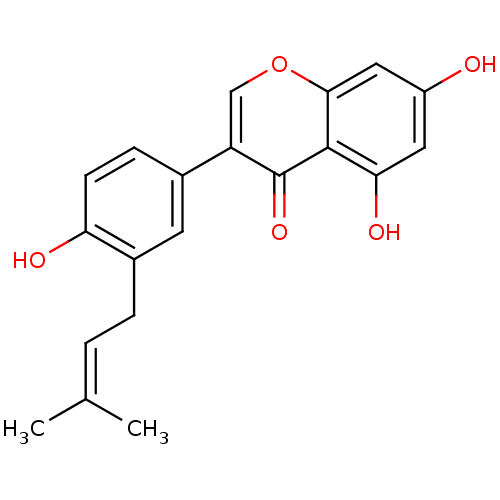
BDBM50349975 CHEMBL1812593
SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-c1coc2cc(-[#8])cc(-[#8])c2c1=O
InChI Key InChIKey=LQEAXLGJZPBTMT-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50349975
Found 3 hits for monomerid = 50349975
Affinity DataIC50: 2.48E+5nMAssay Description:Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad...More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+5nMAssay Description:Inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+5nMAssay Description:Inhibition of influenza A virus H9N2 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrateMore data for this Ligand-Target Pair