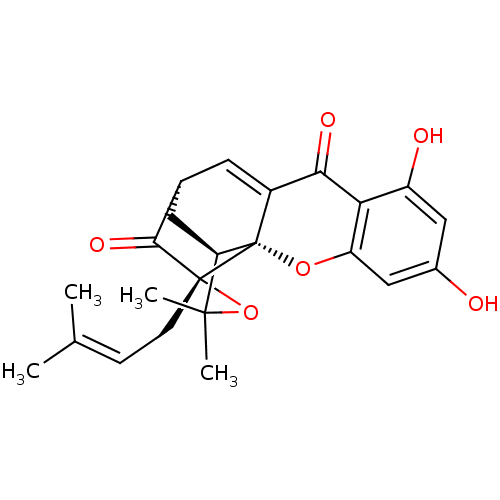
BDBM50346333 (1S,3aR,5R,12aR)-3,3a,4,5-tetrahydro-8,10-dihydroxy-3,3-dimethyl-1-(3-methyl-2-buten-1-yl)-1, 5-methano-1H,7H-furo[3,4-d]xanthene-7,13-dione::CHEMBL1782238
SMILES [#6]\[#6](-[#6])=[#6]\[#6][C@]12[#8]C([#6])([#6])[#6@H]3-[#6]-[#6@H](-[#6]=[#6]4-[#6](=O)-c5c(-[#8])cc(-[#8])cc5-[#8][C@]134)-[#6]2=O
InChI Key
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50346333
Found 2 hits for monomerid = 50346333
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
The Ohio State University
Curated by ChEMBL
The Ohio State University
Curated by ChEMBL
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli assessed as reduction in p-nitrophenol formation using p-nitr...More data for this Ligand-Target Pair