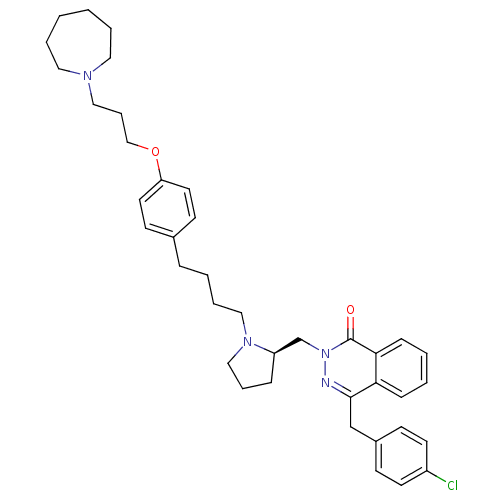
BDBM50341447 4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(hexahydro-1H-azepin-1-yl)propyl]oxy}phenyl)butyl]-2-pyrrolidinyl}methyl)-1(2H)-phthalazinone::CHEMBL1767164
SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1
InChI Key InChIKey=YANGEESWIGIKOP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50341447
Found 21 hits for monomerid = 50341447
Affinity DataIC50: 50.1nMAssay Description:Displacement of labeled dofetilide human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 50.1nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHOK1 cell membranes incubated for 4 hrs in dark by luminescent assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataKd: 0.794nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox...More data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataKi: 15.8nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 31.6nMAssay Description:Antagonist activity at human adrenergic alpha1B receptor expressed in rat fibroblasts by by plate-based calcium imagingMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux...More data for this Ligand-Target Pair
Affinity DataKi: 39.8nMAssay Description:Antagonist activity at human adrenergic alpha1A receptor expressed in rat fibroblasts by by plate-based calcium imagingMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux...More data for this Ligand-Target Pair