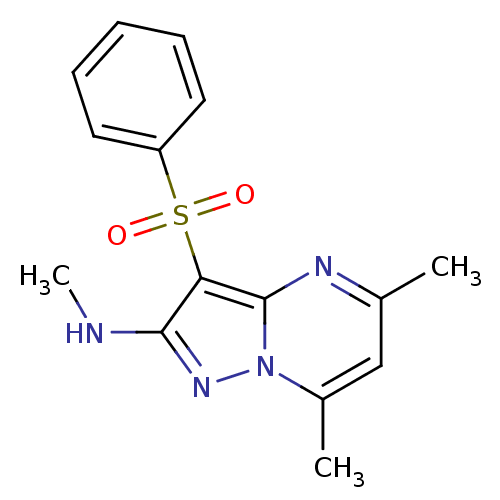
BDBM50340725 CHEMBL1668584::N,5,7-trimethyl-3-(phenylsulfonyl)pyrazolo[1,5-a]pyrimidin-2-amine::US8829009, 1.1(1)
SMILES CNc1nn2c(C)cc(C)nc2c1S(=O)(=O)c1ccccc1
InChI Key InChIKey=YPVOSIDECMYMTD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50340725
Found 8 hits for monomerid = 50340725
Affinity DataIC50: 5nMAssay Description:Determination of antagonistic activity of compounds of general formula 1 towards 5-HT6 receptors. Compounds of general formula 1 were tested for thei...More data for this Ligand-Target Pair
Affinity DataIC50: 5.13E+3nMAssay Description:Displacement of [3H]lysergic acid diethylamide from human recombinant 5HT2B receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Chemical Diversity Research Institute
Curated by ChEMBL
Chemical Diversity Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at human ERG by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]lysergic acid diethylamide from human recombinant 5HT6 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.257nMAssay Description:Antagonist activity at human recombinant 5HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation after ...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Antagonist activity at human recombinant 5HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced cAMP production after 2 ...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Inhibition of human recombinant 5-HT6 receptor expressed in human HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataKi: 1.04E+3nMAssay Description:Antagonist activity at human 5HT2B receptor expressed in HEK293 cells assessed as inhibition of alpha-Me-serotonin-induced intracellular calcium mobi...More data for this Ligand-Target Pair