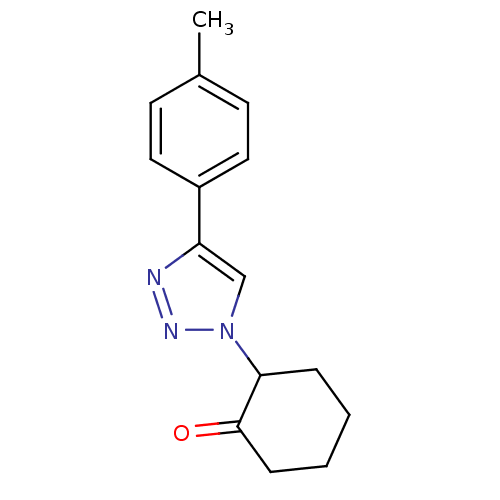
BDBM50334629 2-(4-p-tolyl-1H-1,2,3-triazol-1-yl)cyclohexanone::CHEMBL1642294
SMILES Cc1ccc(cc1)-c1cn(nn1)C1CCCCC1=O
InChI Key InChIKey=GYLRZQXVSISSMM-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50334629
Found 4 hits for monomerid = 50334629
TargetProto-oncogene tyrosine-protein kinase Src(Human)
Institute of Technology and Science
Curated by ChEMBL
Institute of Technology and Science
Curated by ChEMBL
Affinity DataIC50: 3.39E+4nMAssay Description:Inhibition of c-Src after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CckMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human activated LckMore data for this Ligand-Target Pair