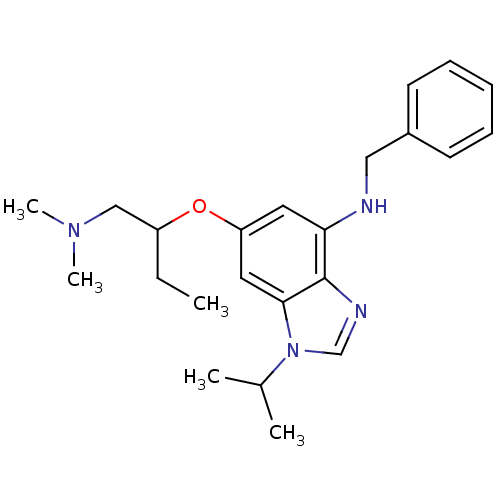
BDBM50334339 CHEMBL1358008::N-Benzyl-6-(1-(dimethylamino)butan-2-yloxy)-1-isopropyl-1H-benzo[d]imidazol-4-amine::cid_44143056
SMILES CCC(CN(C)C)Oc1cc(NCc2ccccc2)c2ncn(C(C)C)c2c1
InChI Key InChIKey=YHLSWJLBOMKEOE-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50334339
Found 4 hits for monomerid = 50334339
Affinity DataIC50: 3.74E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C...More data for this Ligand-Target Pair
TargetApoptotic protease-activating factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 7.98E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C...More data for this Ligand-Target Pair
TargetApoptotic protease-activating factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CDK5/P25 GST-fusion protein assessed as substrate histone H1 phosphorylation by scintillation countingMore data for this Ligand-Target Pair