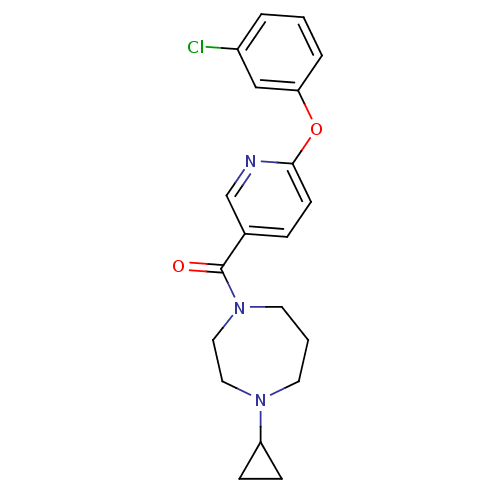
BDBM50321470 (6-(3-chlorophenoxy)pyridin-3-yl)(4-cyclopropyl-1,4-diazepan-1-yl)methanone::CHEMBL1171001
SMILES Clc1cccc(Oc2ccc(cn2)C(=O)N2CCCN(CC2)C2CC2)c1
InChI Key InChIKey=SASIOZSVIDJSAI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50321470
Found 3 hits for monomerid = 50321470
TargetHistamine H3 receptor(Rat)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKd: 11.5nMAssay Description:Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Rat)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 75nMAssay Description:Binding affinity to histamine H3 receptor in rat brainMore data for this Ligand-Target Pair