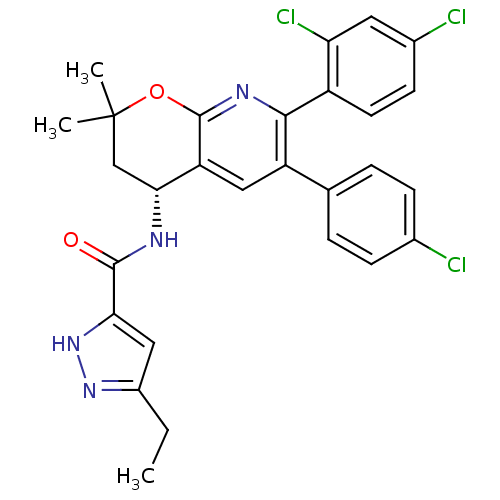
BDBM50316523 CHEMBL1095789::N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-dichlorophenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-4-yl]-5-ethyl-1H-pyrazole-3-carboxamide
SMILES CCc1cc([nH]n1)C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1
InChI Key InChIKey=QRLAIUSGKUVHBM-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50316523
Found 3 hits for monomerid = 50316523
Affinity DataIC50: 2.10nMAssay Description:Displacement of [3H]CP-55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Displacement of [3H]CP-55940 from human recombinant cannabinoid CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair