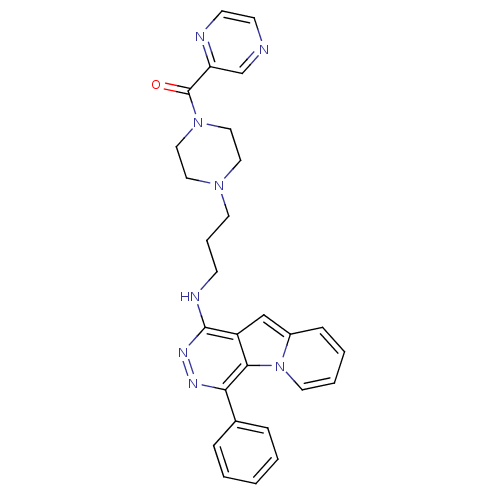
BDBM50314558 (4-(3-(4-phenylpyridazino[4,5-b]indolizin-1-ylamino)propyl)piperazin-1-yl)(pyrazin-2-yl)methanone::CHEMBL1090016
SMILES O=C(N1CCN(CCCNc2nnc(-c3ccccc3)c3c2cc2ccccn32)CC1)c1cnccn1
InChI Key InChIKey=UBFOHSDZACWGDX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50314558
Found 4 hits for monomerid = 50314558
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of PDE4B3 expressed in CHO cells assessed as isoproterenol-induced [125I]cAMP accumulation after 15 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PDE4D4 expressed in CHO cells assessed as isoproterenol-induced [125I]cAMP accumulation after 15 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Displacement of [methyl-3H]rolipram from PDE4B3 expressed in CHO cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.37E+3nMAssay Description:Displacement of [methyl-3H]rolipram from PDE4D4 expressed in CHO cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair