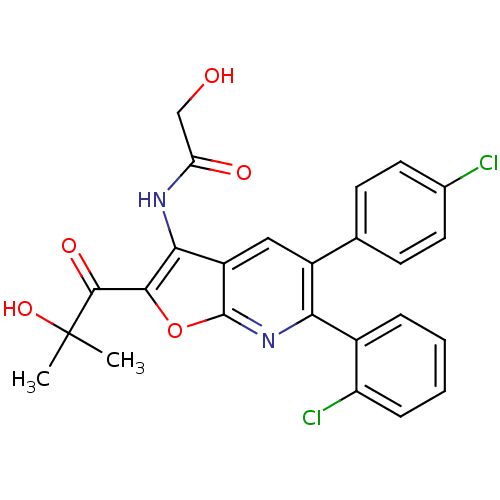
BDBM50314108 CHEMBL1092169::N-(6-(2-chlorophenyl)-5-(4-chlorophenyl)-2-(2-hydroxy-2-methylpropanoyl)furo[2,3-b]pyridin-3-yl)-2-hydroxyacetamide
SMILES CC(C)(O)C(=O)c1oc2nc(-c3ccccc3Cl)c(cc2c1NC(=O)CO)-c1ccc(Cl)cc1
InChI Key InChIKey=NLFVFVQFPLEKDA-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50314108
Found 10 hits for monomerid = 50314108
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of human ERG potassium channelMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of human CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of cannabinoid CB1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of cannabinoid CB2 receptorMore data for this Ligand-Target Pair