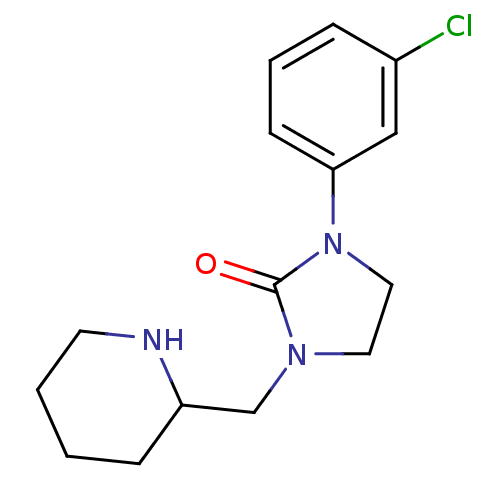
BDBM50313618 1-(3-chlorophenyl)-3-(piperidin-2-ylmethyl)imidazolidin-2-one::CHEMBL1085672
SMILES Clc1cccc(c1)N1CCN(CC2CCCCN2)C1=O
InChI Key InChIKey=HVSRNYNPOUFDNJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50313618
Found 3 hits for monomerid = 50313618
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant CYP2D6 by spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94E+3nMAssay Description:Displacement of [3H]dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Activity at histamine H1 receptorMore data for this Ligand-Target Pair