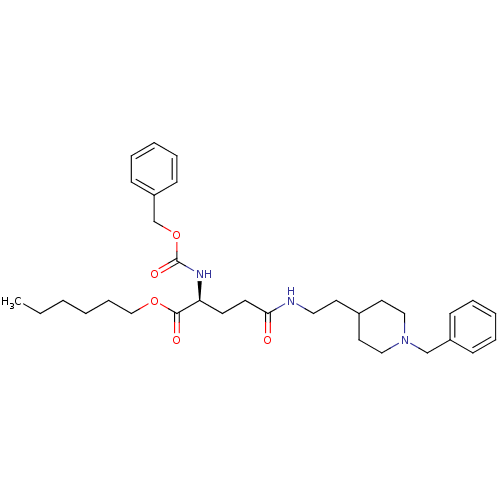
BDBM50311995 (S)-hexyl 2-(benzyloxycarbonylamino)-5-(2-(1-benzylpiperidin-4-yl)ethylamino)-5-oxopentanoate::CHEMBL1082082
SMILES CCCCCCOC(=O)[C@H](CCC(=O)NCCC1CCN(Cc2ccccc2)CC1)NC(=O)OCc1ccccc1
InChI Key InChIKey=MHODVJVESFLWQL-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50311995
Found 4 hits for monomerid = 50311995
Affinity DataIC50: 250nMAssay Description:Inhibition of human AChE in erythrocyteMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of AChE in bovine erythrocyte by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of BuChE in horse serum by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 730nMAssay Description:Inhibition of human BuChE in serumMore data for this Ligand-Target Pair