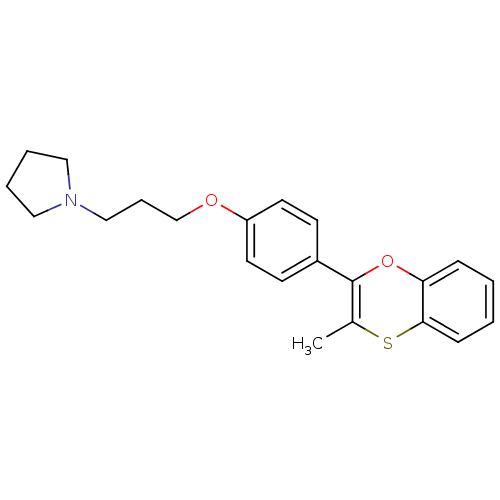
BDBM50296186 1-(3-(4-(3-methylbenzo[b][1,4]oxathiin-2-yl)phenoxy)propyl)pyrrolidine::CHEMBL560923
SMILES CC1=C(Oc2ccccc2S1)c1ccc(OCCCN2CCCC2)cc1
InChI Key InChIKey=DREYZGCIOLBBDK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50296186
Found 4 hits for monomerid = 50296186
Affinity DataIC50: 8.60nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding by ce...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assayMore data for this Ligand-Target Pair