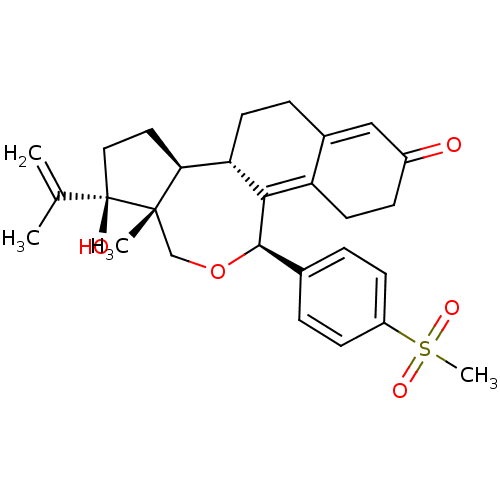
BDBM50295636 (1R,3S,3aR,3bS,10R)-1-Hydroxy-1-isopropenyl-10-(4-methanesulfonyl-phenyl)-12a-methyl-2,3,3a,3b,4,5,9,10,12,12a-decahydro-1H,8H-11-oxa-naphtho[2,1-e]azulen-7-one::CHEMBL561863
SMILES CC(=C)[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](OC[C@]12C)c1ccc(cc1)S(C)(=O)=O
InChI Key InChIKey=RPHZKNSCEWZAMO-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50295636
Found 2 hits for monomerid = 50295636
TargetGlucocorticoid receptor(Human)
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Antagonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of corticoid-induced transcription after 16 hrs by glucocor...More data for this Ligand-Target Pair
TargetProgesterone receptor(Human)
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair