BDBM50286772 CHEMBL4160841
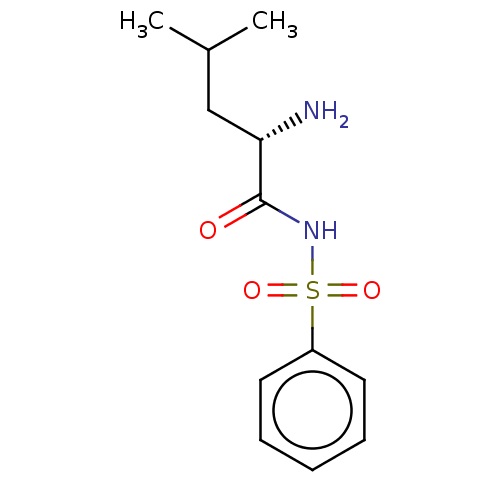
SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1ccccc1
InChI Key InChIKey=HBYIBLSAKVSPSZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50286772
Found 4 hits for monomerid = 50286772
Affinity DataKd: 1.30nMAssay Description:Binding affinity to Escherichia coli LeuRS by isothermal titration calorimetric calorimetryMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase(Staphylococcus aureus (strain NCTC 8325 / PS 47))
Oxford Drug Design
Curated by ChEMBL
Oxford Drug Design
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of Staphylococcus aureus LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumptionMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumptionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human LeuRS assessed as reduction in ATP consumptionMore data for this Ligand-Target Pair