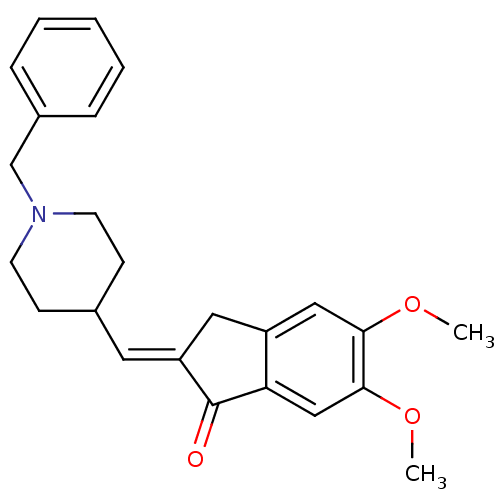
BDBM50280646 2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5,6-dimethoxy-indan-1-one::CHEMBL168938
SMILES COc1cc2c(cc1OC)C(=O)/C(=C/C3CCN(CC3)Cc4ccccc4)/C2
InChI Key InChIKey=LPMOTUSFDTTWJL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50280646
Found 8 hits for monomerid = 50280646
Affinity DataIC50: 13nMAssay Description:Inhibition of Acetylcholinesterase activity in mouse brain homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of mouse BACE-1 preincubated for 10 mins followed by fluorescent peptide substrate addition measured for 30 mins by fluorescence plate rea...More data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.74E+3nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 697nMAssay Description:Inhibition of BACE-1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Non-competitive inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 2.44E+3nMAssay Description:Mixed inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair