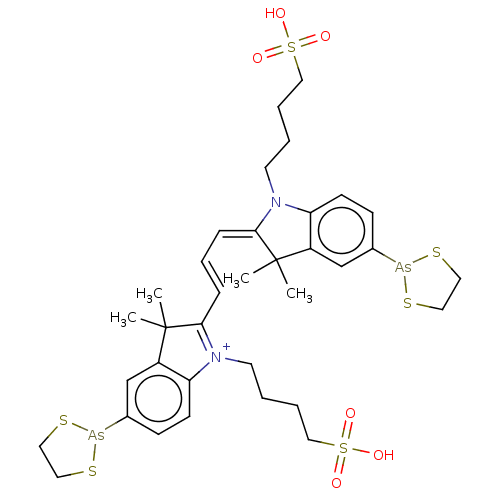
BDBM50272021 CHEMBL4128743
SMILES CC1(C)\C(=C/C=C/C2=[N+](CCCCS(O)(=O)=O)c3ccc(cc3C2(C)C)[As]2SCCS2)N(CCCCS(O)(=O)=O)c2ccc(cc12)[As]1SCCS1
InChI Key InChIKey=UDHHTMYURAAYGN-UHFFFAOYSA-O
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50272021
Found 2 hits for monomerid = 50272021
Affinity DataIC50: 125nMAssay Description:Inhibition of allosterically sensitized His6-tagged human PTP1B catalytic domain P87C/A122C mutant using pNPP as substrate incubated for 90 mins by q...More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Inhibition of allosterically sensitized His6-tagged human PTP1B catalytic domain P87C/A122C mutant overexpressed in Escherichia coli crude cell lysat...More data for this Ligand-Target Pair