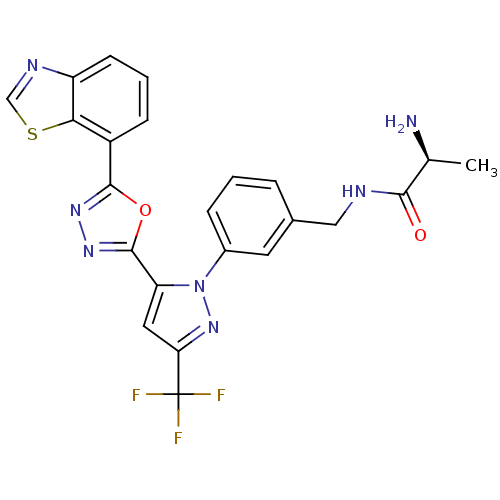
BDBM50258790 (S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,4-oxadiazol-2-yl)-1H-pyrazol-1-yl)benzyl)propanamide::(S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,4-oxadiazol-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzyl)propanamide::CHEMBL468927
SMILES C[C@H](N)C(=O)NCc1cccc(c1)-n1nc(cc1-c1nnc(o1)-c1cccc2ncsc12)C(F)(F)F
InChI Key InChIKey=KODBDIFHMBDFOH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50258790
Found 9 hits for monomerid = 50258790
TargetProtein arginine N-methyltransferase 3 [N508S](Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of PRMT3 by methylation assayMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of CARM1 assessed as blockade of histone H3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >2.50E+4nMAssay Description:Inhibition of human PXRMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4 expressed in human hepatocytesMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2C9 expressed in human hepatocytesMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2C19 expressed in human hepatocytesMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2D6 expressed in human hepatocytesMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 1 [11-371](Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of PRMT1 by methylation assayMore data for this Ligand-Target Pair